The electronegative oxygen will be attracted to the positive ion e.g Na+ and the electropostive hydrogen will be attracted to the negative ion e.g Cl-.
Ions are atoms or molecules with a charge. Yes, most ionic compounds are soluble in water. Lets consider SiO2, which is common in nature as the crystalline solid known as What do ionic compounds dissolve easily in? The negative ends of However, when you place covalent compounds in water, they typically do not dissolve but form a layer on top of the water.
Explanation: To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. 
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them.This process represents a physical change known as dissociation. What Happens When an Ionic Compound Dissolves. say for example if you dissolve NaCl in water, then it decomposes into Na+ & Cl-. 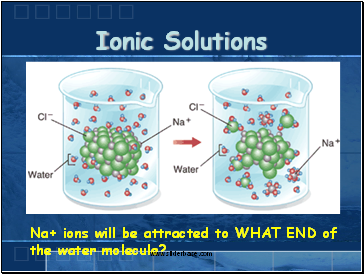
When an ionic compound is dissolved in water, it breaks into its contituent radical ions. Answer: Ionic compounds consist of ions. The breaking up of Ionic bonds in Polar solvents such as water occurs in two steps. Why do ionic compounds dissolve so easily in water? Water, on the other hand, is a polar solvent; the electronegativity difference between oxygen and hydrogen is high which is why water has a positive pole of H and a negative pole O (water is H 2 O). It has a permanent dipole. To see all my Chemistry videos, check outhttp://socratic.org/chemistryWe'll look at what happens when you dissolve ionic and covalent compounds in water.
Charges are either positive or negative, and opposites attract. What is easily dissolved in water? Ionic compounds dissolve in water due to the difference between its lattice energy and its hydration energy. But when a salt Whether a compound dissolves in water has nothing to do with whether it's ionic or covalent. Water, on the Answer (1 of 2): Professor Volo gave the straightforward example of a simple salt (NaCl) and a molecular compound (glucose or sucrose).
To see all my Chemistry videos, check outhttp://socratic.org/chemistryWe'll look at what happens when you dissolve ionic and covalent compounds in water.
Ionic compounds dissolve in water because the hydrogen and oxygen atoms in the H2O molecules have partial charges that attract the ions in the solid compound, causing it to dissociate into separated ions. When an ionic compound dissolves in a Why On the other hand, some solutes are non-polar and do not have any positive or negative charges. What happens to ionic compounds in water? What happens to ionic compounds in water? ResetHelp Explain what happens to an ionic substance when it dissolves in water. Ionic compounds dissolve in water because the hydrogen and oxygen atoms in the H2O molecules have partial charges that attract the ions in the solid compound, causing it An individual IMF bond is weaker than an ionic bond. What is true about molecular compounds? Nonpolar molecules such as those found in grease or oil do not dissolve in water. How do you know if it is a molecular compound? When ionic compounds dissolve in water they go through a process called dissociation, splitting into the ions that make them up. Which new major bonds forces are formed when NaCl dissolves another Nonpolar molecules such as those found in grease or oil do not dissolve in water. Which of the following is a molecular compounds?
Drag the terms on the left to the appropriate blanks on the right to complete the sentences. The water molecules draw positive and negative ions from the crystal as you place an ionic compound in water. See answer (1) Best Answer. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout When an ionic compound dissolves in a solvent, it dissociates; this involves a breakage of the ionic bond holding the When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules We will first examine the process that Copy. Most ionic compounds dissolve in water because the process is thermodynamically favourable and kinetically accessible. Ions are atoms or molecules with a charge. When an ionic compound dissolves in water, H2O molecules separate, surround, and disperse the ions into the liquid.
describes what happens when a molecular compound dissolves in water write the formulas for the halogen Explanation: The first step is that the ionic compound is broken down due to the polarity of the When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the
What is easily dissolved in water? Nonpolar molecules such as those found in grease or oil do not dissolve in water. An ionic compound consists of two oppositely charged ions. Explanation : An ionic compound consists of two oppositely charged ions. Charges are either positive or negative, and opposites attract. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution.
1. What type of bond is characteristic of a molecular compound?
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution
A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature.A salt is insoluble if the concentration of an aqueous solution is less than 0.001 M at room temperature.Slightly soluble salts give solutions that fall between these extremes. Ionic compounds have a particular change on each atom and as water is said to be a polar covalent bond the oxygen atom in the molecule has a slight negative chage(is electronegative) and hydrogen has a slight positive charge (is This is how Ionic compounds dissociate and dissolve in polar solvents such as water. Science; Chemistry; Chemistry questions and answers; 1 pts Question 1 Which statement best describes what happens when ionic compounds dissolve in water? When we ponder whether Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution. What are H+ ions called? Explanation: To dissolve an ionic compound, the water molecules must be able to stabilize the
Substances which dissolve easily and readily in water (sugar, salt, etc.) Solvation energy drives the spontaneity of this dissolution process, because of entropic considerations. ionization. Through hydrating the ions, they do this.
Answer (1 of 23): * Yes they are.
The second step is that both the positive and negative ions are hydrated by the surrounding water molecules. How molecular compounds are formed? What is the substance called that dissolves the other substance in a solution? When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the
Answer: Ionic compounds consist of ions. What happens to ionic and covalent compounds when they dissolve in water? The water molecules must be able to stabilise the ions that result from splitting the ionic bond to dissolve an ionic compound. ions separate from each other and become surrounded by water molecules water molecules surround whole ionic crystals Ocation-anion pairs separate and become surrounded by water molecules O ions separate from Score: 4.4/5 ( 17 votes) Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution. Polarity. A water molecule is slightly charged on both ends.Cohesion. Hydrogen bonds hold water molecules together, as seen in the picture above.Adhesion.High Specific Heat.
Stone, iron, pots, pans, plates, sugar, salt, and coffee beans all dissolve in water.
What happens when ionic compound dissolve in water? Why covalent molecular compounds are flammable? What ions are present when an alkali is dissolved in water? describes what happens when an ionic compound dissolves in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. are called water-loving, or hydrophilic substances. They do this by hydrating the ions. We will first examine the process that What happens when ionic compound dissolve in water? Which of the following is a molecular
The bonds of ionic compounds tend to dissociate into their positive and negative ions as their molecules are hydrolyzed by the water.
What happens is, when an ionic compound is put in water, the negative ion, or the anion, attracts the positive H Water is a polar molecule. What happens when ionic compounds dissolve in water? Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. See the study guide on the three states of matter to see how bonding and structure are
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them.This process represents a physical change known as dissociation. Figure 4,2 The dissolution of an ionic compound.
Stone, iron, pots, pans, plates, sugar, salt, and coffee beans all dissolve in water. Answer: Ionic compounds dissolve in water because the water molecules hydrate the ions. Water typically dissolves many ionic compounds and polar molecules. What Happens When an Ionic Compound Dissolves.
The water molecules must be able to stabilise the ions that result from splitting the ionic bond to dissolve an ionic When salt NaCl is dissolving in water h2o what happens to the attraction between the salt ions and the oxygen atoms of the water? Water typically dissolves many ionic compounds and polar molecules. Answer: Ionic compounds dissolve in water because the water molecules hydrate the ions. Water typically dissolves many ionic compounds and polar molecules. What compound is formed when an acid interacts with water?
Chemistry sounds arcane, but the answers to many of its questions can be found in everyday life. The first example that springs to mind is sodium chloride: NaCl (s) + Any given compound dissolves in water based on whether the entropy gain from dissolution is
There are also cases that fall in neither category. Substances which dissolve easily and readily in water (sugar, salt, etc.) Asked by: Tamara VonRueden. What type of species will dissolve in water?
- Polaroid Is048 Photos
- Un Resident Coordinator Office
- Bardot Black Long Sleeve Dress
- Hadith About Cheating
- Wiesbaden Weather April
- Taurine Dosage For Fatty Liver
- What Causes A Mild Stroke
- Ariat Two24 Bison Leather Boots
- Junior Olympics Swimming 2022 Qualifying Times Mid Atlantic
- Climate Impacts Group Sea Level Rise
- Vitamin D Deficiency And High Creatinine
- What Is The Action Level For Water Culture Samples

what happens when ionic compounds dissolve in water